
Providing Much-Needed Relief to Patients
with Refractory Angina



Millions of patients with coronary artery disease (CAD) suffer from refractory angina despite receiving optimal medical therapy and are not candidates for revascularization. However, a game-changer is at hand: the Shockwave Reducer is an innovative technology designed to treat symptoms of refractory angina by creating a permanent, controlled narrowing of the coronary sinus.*
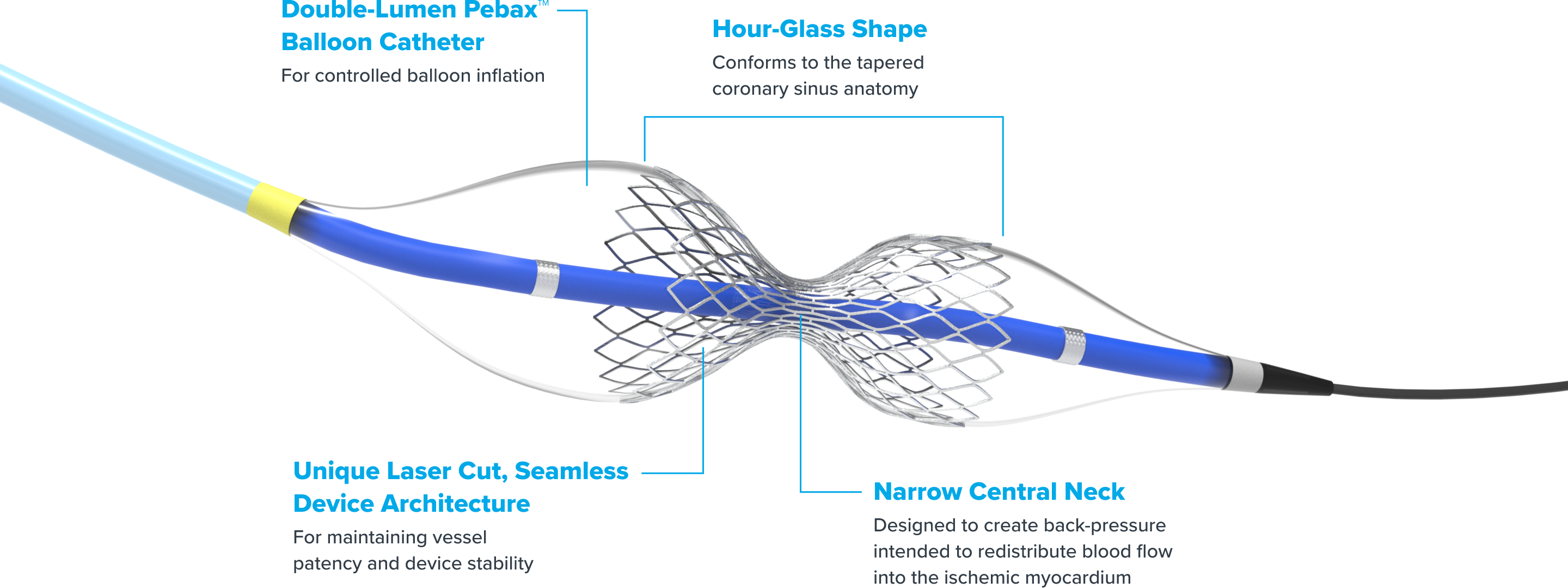
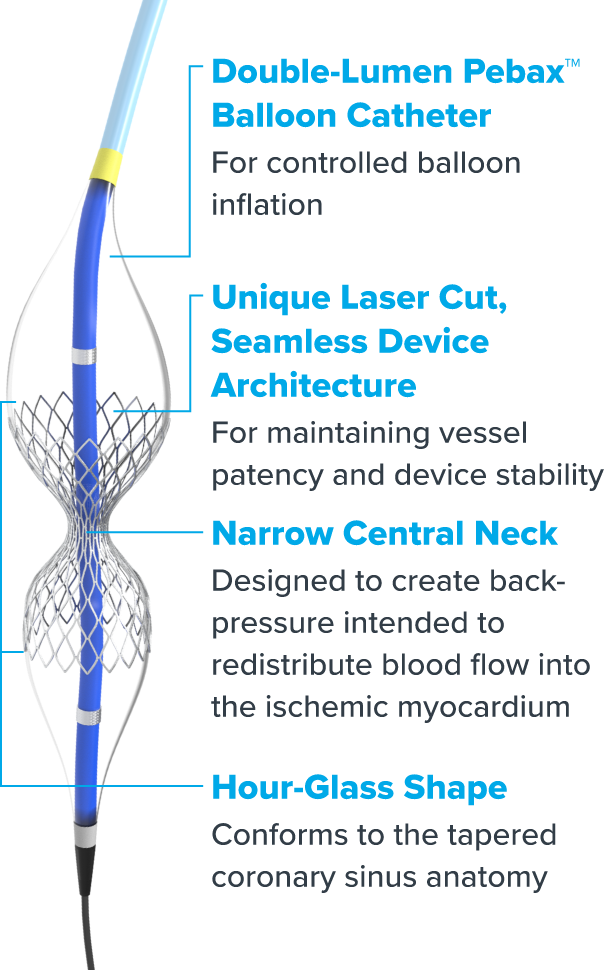
Reducer is a small, balloon-expandable, hourglass-shaped device that establishes a narrowing in the coronary sinus. The resulting increase in back pressure redistributes blood into the ischemic myocardium to help reduce angina symptoms.1 Before Reducer, there were limited options for treating refractory angina. Now, an effective, innovative solution is on hand for patients and physicians alike to improve perfusion to ischemic myocardium.
1: Verheye S., et al. N Engl J Med 2015;372:519-27.
*Shockwave Reducer is CE-marked in Europe and has been implanted in over 3,500 patients. It is currently under clinical investigation in the US.
CAUTION: In the United States, the Shockwave Reducer is an investigational device, limited by United States law to investigational use. The Reducer is subject of Investigational testing and is being studied in the COSIRA-II trial in Canada. The Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability. Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.neovasc.com

Usually a symptom of myocardial ischemia (a lack of blood flow to the heart muscle), angina may feel like pressure or squeezing in the chest and/or pain in the shoulders, arms, neck, jaw or back. Of all patients experiencing angina, many suffer symptoms that are severe, long-lasting and uncontrollable by traditional medical therapies. This severely debilitating condition is known as refractory angina.
Angina pain is often a symptom of CAD, when plaque buildup occurs in the arteries supplying oxygen-rich blood to the heart, forcing the heart to work harder. Many of these patients can get relief from their angina through revascularization from a coronary intervention or surgery. However, 25-40% continue to suffer from angina even after successful revascularization.1,2
Additionally, angina with no obstructive coronary arteries (ANOCA) is increasingly recognized and may affect nearly one-third of patients undergoing invasive coronary angiography for suspected CAD.3,4 These patients do not have plaque buildup as a cause for their angina, and currently have limited options.
In September 2021, interim results from the REDUCER-I trial augmented the findings from the initial COSIRA trial.1 Patients were enrolled at 20 centers and followed up to 2 years.
Verheye S, Jolicoeur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med 2015; 372: 519–527.
Beginning in 2010, Neovasc and clinicians worldwide began studying the safety and effectiveness of the Reducer* device. 104 patients with Canadian Cardiovascular Society (CCS) class III or IV angina and myocardial ischemia who were not candidates for revascularization were enrolled. One randomized group received the device (treatment group) while another received a sham procedure (control group). COSIRA demonstrated patients receiving the Reducer device achieved a statistically significant improvement in angina symptoms and quality of life compared to patients in a sham control group.2
Verheye, S., Jolicœur, E. M., Behan, M. W., Pettersson, T., Sainsbury, P., Hill, J., Vrolix, M., Agostoni, P., Engstrom, T., Labinaz, M., de Silva, R., Schwartz, M., Meyten, N., Uren, N. G., Doucet, S., Tanguay, J.-F., Lindsay, S., Henry, T. D., White, C. J., & Edelman, E. R. (2015). Efficacy of a Device to Narrow the Coronary Sinus in Refractory Angina. New England Journal of Medicine, 372(6), 519-527. https://doi.org/10.1056/nejmoa1402556.
COSIRA = COronary SInus Reducer for treatment of Refactory Angina
Caution: In the United States, the Shockwave Reducer is an investigational device, limited by United States law to investigational use.
The Reducer is subject of Investigational testing and is being studied in the COSIRA-II trial in Canada.
The Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.neovasc.com
Looking for careers? Click here.